In complex organisms like mammals to keep all the cellular as well as physiological processes going, communication between cells, tissues and organs is of paramount importance. This communication is achieved because of specific molecules known as hormones. Similar to the mammalian system plants also produce chemical signals to maintain homeostasis between growth, morphogenesis and regulation of metabolic processes. The first idea of such chemical signal molecules in plants originated in the 19th century with German scientist Julian Von Sachs. These chemical messengers in plants are particularly termed Phytohormones. In this article, we will talk about the different naturally occurring plant hormones and their function in plant growth and metabolism.
Generally, about five naturally occurring plant hormones are present in plants which are as follows:
- Auxin
- Gibberellins
- Abscisic acid
- Cytokinin
- Ethylene
Auxins
Auxin is the very first hormone discovered in plants. The foundation for the discovery of auxins dates back to the 19th century when Charles Darwin and his son Francis Darwin were studying the bending of the coleoptile region of grass (Phalaris canariensis) towards the light a phenomenon popularly known as phototropism. Darwin found that the tip of the coleoptile perceived the light but when covered with foil the coleoptile would not bend. But, the region responsible for the bending of the coleoptile is called growth is located several millimetres below the coleoptile region, hence Darwin concluded that some kind of signal is produced in the tip region which diffuses to the growth zone and causes the bending of the coleoptile. In 1926, Frits Went reported the presence of chemicals responsible for the growth of the coleoptile in the tip of oat Avena sativa coleoptile. Went allowed the chemical to diffuse out in the gelatin blocks. He also observed that when these blocks were placed on the decapacitated coleoptile regions they had the capacity to cause bending in absence of the light source. As the compound had the capability of the elongation of the coleoptile region the compound was named Auxin from the Greek auxin, meaning “to increase” or “to grow.”
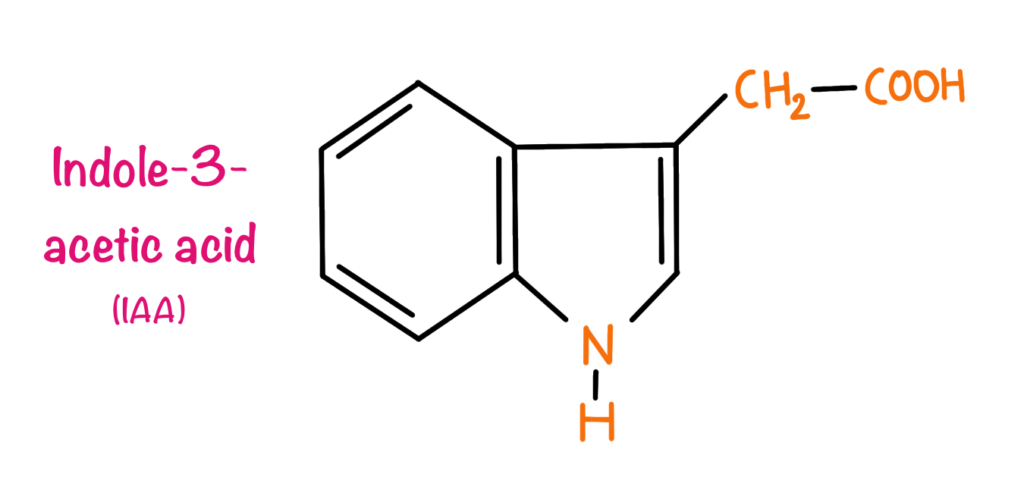
All the auxins have indole rings in their structure. Indole-3-acetic acid is the only naturally occurring auxin in plants. Other synthetic analogues include Dicamba,2,4-Dichlorophenoxyacetic acid (2,4-D), 4-Chloroindole-3-acetic acid, Indole-3-butyric acid.
The functions of auxins in plants include cell elongation. Auxins also promote growth in stems and coleoptiles and inhibit growth in roots.
Gibberellins
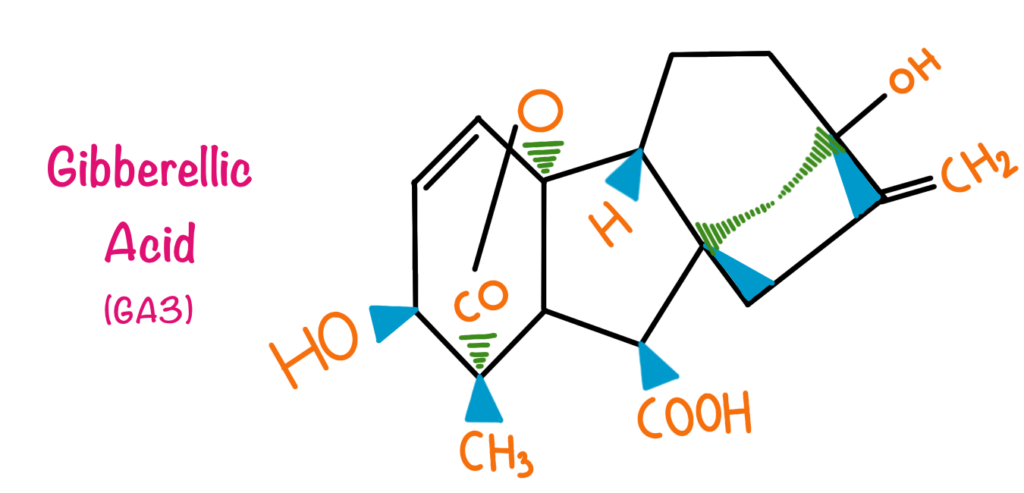
Gibberellins are the second group of plant hormones that are characterized. Gibberellins contain about 125 different types of compounds. Chemically Gibberellins can be termed as a group of tetracyclic diterpenes. Gibberellins are 20 carbon compounds but, in some compounds, the carbon number is reduced to 19 due to the loss of 1 carbon to the lactone ring in metabolism.
The Discovery of the Gibberellins dates back to the year 1926 when Japanese botanist Elichi Kurosawa was investigating ‘bakanae’ popularly known as ‘Foolish seedling disease of rice’ which is caused by fungus Gibberella fujikuroi. The disease caused excessive stem elongation and yellow ribs in leaves. The scientist observed that the excessive elongation of stem and the yellowing of ribs is particularly due to the compound secreted by the fungus infecting tall plants. This compound was given the name Gibberellins. In 1930, the compound was characterized by two scientists Teijiro Yabuta and Takeshi Hayashi. Later on, 125 different Gibberellic acids were characterized, 12 specifics to G. fujikuroi, 100 specific in higher plants and 13 ubiquitous.
Gibberellins are particularly involved in stem elongation in plants. In some cases, they are known to induce flowering. Gibberellins prevent leaf senescence and fruit senescence, because of this property they are actively used in grape plantations. In the malting industry, gibberellins are added in barley, this is because the aleurone layer in barley has amylases and other enzymes which are triggered by gibberellins. This speeds up the malting process. Due to the large commercial applications of the gibberellic acids, they are produced commercially by the process of fermentation.
Abscisic acid (ABA)
Seeds and buds of plants remain in a non-growth condition until environmental factors are favorable for them, this process is known as dormancy. Efforts have been made to understand the process of dormancy. Plant physiologists believed that dormancy is due to the presence of some inhibitory compounds. Many scientists tried to isolate these compounds from dormant seeds and buds. In 1960, labs in the USA reported an Abscission accumulating compound from young cotton fruit and named it Abscisin II. Similarly, labs in the United Kingdom found a compound that accelerated dormancy and named it Dormin II. In 1965, F. Adiccotts labs found that the Dormin II is very similar to Abscisin II and named it Abscisic acid.
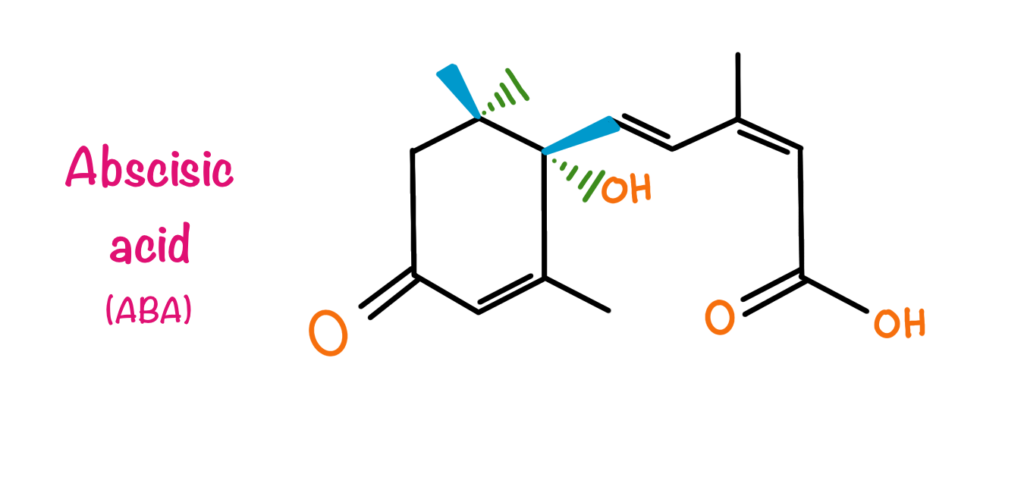
In vascular plants, Abscisic acid is a ubiquitous plant hormone. It has been detected in every major plant tissue from root cap to apical bud. ABA is a 15-carbon compound whose structure is very similar to some carotenoid molecules. ABA has two isomers cis and trans depending upon the position of the terminal carboxylic group. All the naturally occurring ABA are in the cis conformation. Due to the presence of asymmetric carbon atoms at the primary position, ABA has S and R enantiomers. S enantiomer is the naturally occurring one. The commercially available product is a racemic mixture of both the S and R enantiomers. Later on, it was found that the main function due to which the hormone got its name i.e., Abscission is due to the ethylene but ABA can induce the production of ethylene. ABA is known to inhibit growth and stomatal opening particularly when the plant is under a stress. ABA also regulates seed dormancy and maturation in plants.
Cytokinin
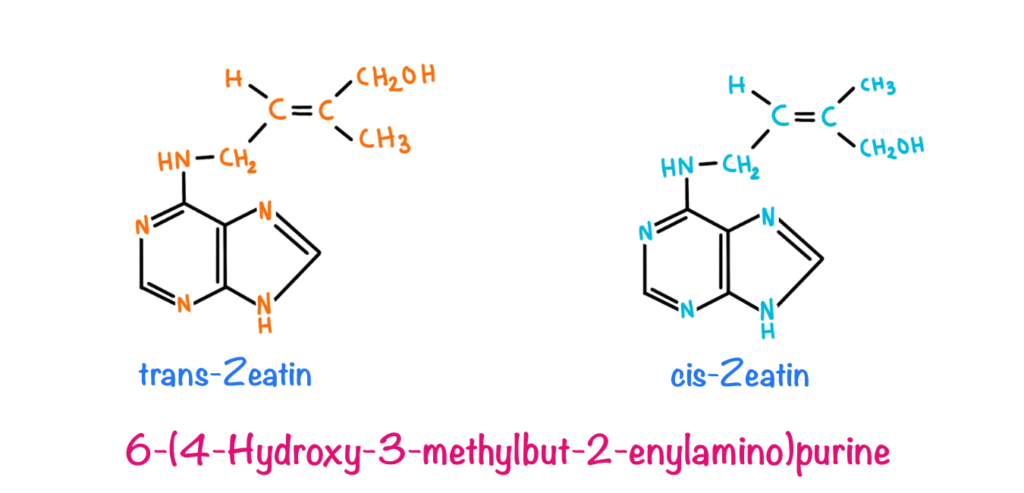
The Discovery of cytokinin was done when scientists were trying to discover a compound responsible for the cell division and proliferation in plants. Van Overbeek (1941) and Caplin and Steward (1948) added raw coconut water in a medium that supported the growth of immature embryos of datura. Later on, in 1974 it was discovered by Letham et al that the compound supporting the growth of embryos from coconut water is Ribosyl zeatin. Miller and Skoog identified 6-furfuryl aminopurine from herring’s sperm DNA, which was found to be a synthetic analogue of ribosyl zeatin. These compounds were later on classified under a common group known as cytokines.
Zeatin is the most abundant naturally occurring cytokine. Typically, a cytokine includes an Adenine molecule along with an isopentyl side chain and N6 amino acid chain. Naturally occurring cytokines include Zeatin, Ribosyl zeatin and N6-(Δ2-Isopentenyl)-adenine (IP) while synthetic analogues include Benzyl adenine, Kinetin and Benzyl amino pentin.
Cytokinin from their discovery have been known to carry out many physiological and developmental processes but control of cell division is the main role of this hormone. Other processes regulated by cytokine include leaf senescence, nutrient mobilization, apical dominance, the formation and activity of shoot apical meristems, floral development, the breaking of bud dormancy, and seed germination. Cytokinin also appear to mediate many aspects of light-regulated development, including chloroplast differentiation, the development of autotrophic metabolism, and leaf and cotyledon expansion.
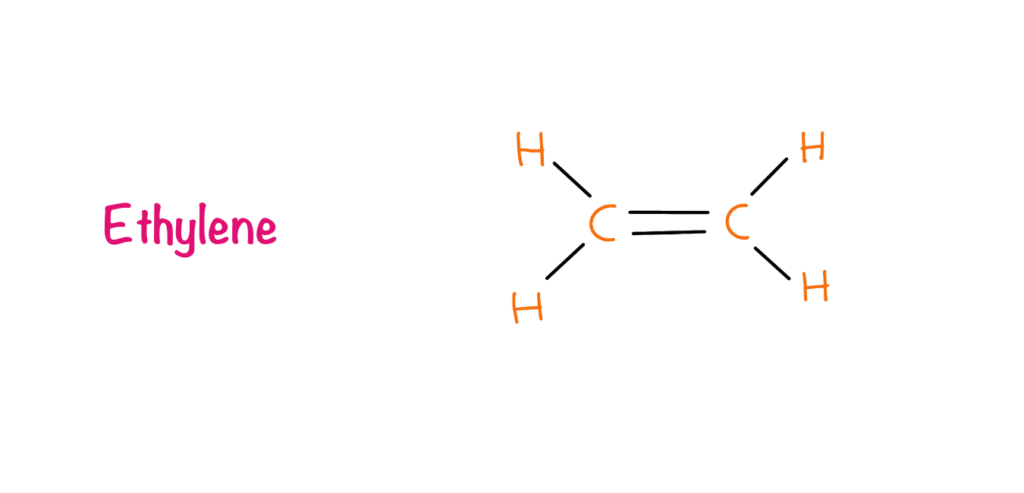
Ethylene
In 1901, a graduate student from Botanical Institute of St. Petersburg in Russia, Dimitry Neljubov observed that dark-grown pea seedlings showed the symptoms of triple response i.e. reduced stem elongation, increased lateral growth (swelling), and abnormal, horizontal growth. When the same plants were grown in natural conditions the growth was regained normally. Neljubov reported the effect is due to the gas ethylene which is present in the laboratory due to the coal gas. In 1934, R. Gane et al identified ethylene as a natural plant product and owing to its dramatic effect on plants it was classified as a hormone.
Ethylene is produced by all parts of higher plants. Meristematic and nodal regions are the most active regions of ethylene production. It was found that ethylene production increases drastically during fruit ripening, leaf abscission and flower senescence. Stresses are also responsible for the production of ethylene such as high temperatures, low temperatures, drought stress, biotic stresses etc. The pathway of the ethylene production includes methionine as the precursor and ACC (1-aminocyclopropane-1-carboxylic acid) serves as an intermediate.