When the world is suffering from a pandemic of Covid-19, the only hope to combat the situation is effective production and worldwide vaccination. In the previous articles, we discussed vaccines, their types, and their mode of action; in this article, we will focus on vaccines that are developed and are being developed across the globe to combat the current situation. So first, let us take into consideration major vaccine candidates who are being used worldwide.
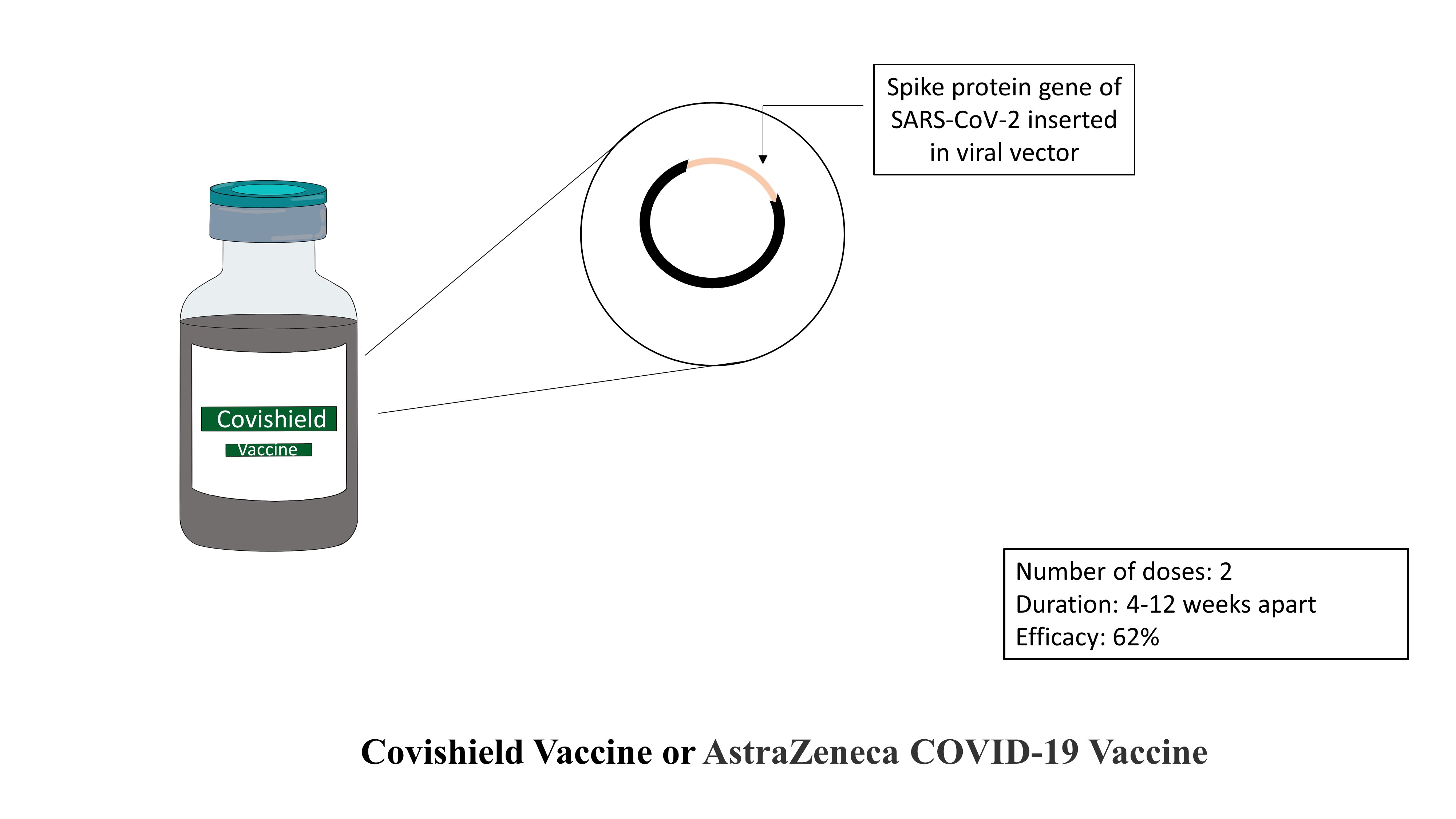
Covishield Vaccine or AstraZeneca COVID‐19 Vaccine:
AstraZeneca COVID‐19 Vaccine (manufactured by AstraZeneca) and Covishield Vaccine (manufactured by Serum Institute of India) falls under the recombinant vector vaccine category. The vaccine is developed jointly by AstraZeneca and Oxford Institute. The vaccine mainly consists of a gene coding for a spike protein of SARS-COV-2 virus inserted in a Chimpanzee Adenoviral Vector. Chimpanzee Adenoviral Vector is an entirely safe vector that does not cause any disease. Once inside the host, gene coding for the coat protein of SARS-COV-2 is transcribed and translated to produce many coat proteins. These coat proteins act as antigenic proteins against which the immune response is generated. The vaccine needs to be taken in two doses. The second dose must be taken after 4-12 weeks of the first dose. The typical dosage for the vaccine is 0.5mL via an intramuscular injection. Extensive studies and controlled trials have reported the vaccine’s efficacy to be 62% after the completion of both doses.
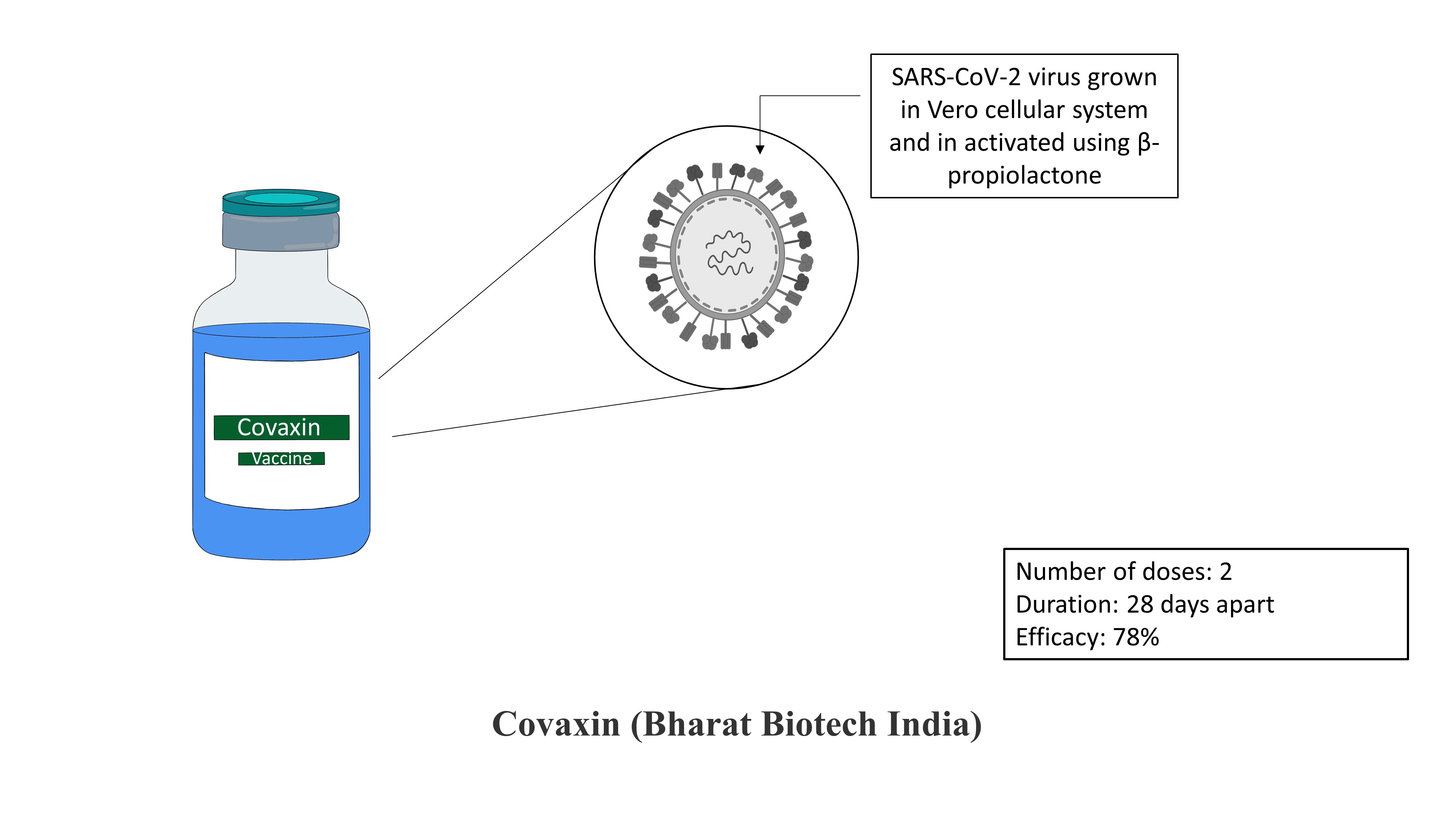
Covaxin (Bharat Biotech India):
Covaxin is a vaccine manufactured by Bharat Biotech, India. The vaccine is developed by the Indian Council of Medical Research-National Institute of Virology along with Bharat Biotech, India. The vaccine is an inactivated vaccine developed by growing the SARS-COV-2 virus in Vero cells. These Vero cells are also known as African green monkey kidney cells. Vero cells are the cells recognized by World Health Organization (WHO) and are previously used to produce many vaccines such as rotaviral vaccines, influenza vaccines etc. The inactivation is carried out by using β-propiolactone. As the virus is inactivated, the vaccine is safe, and there is no possibility of the virus reverting and causing infection. The vaccine is given in 2 doses intramuscularly. The vaccine is 78% effective after the second dose.
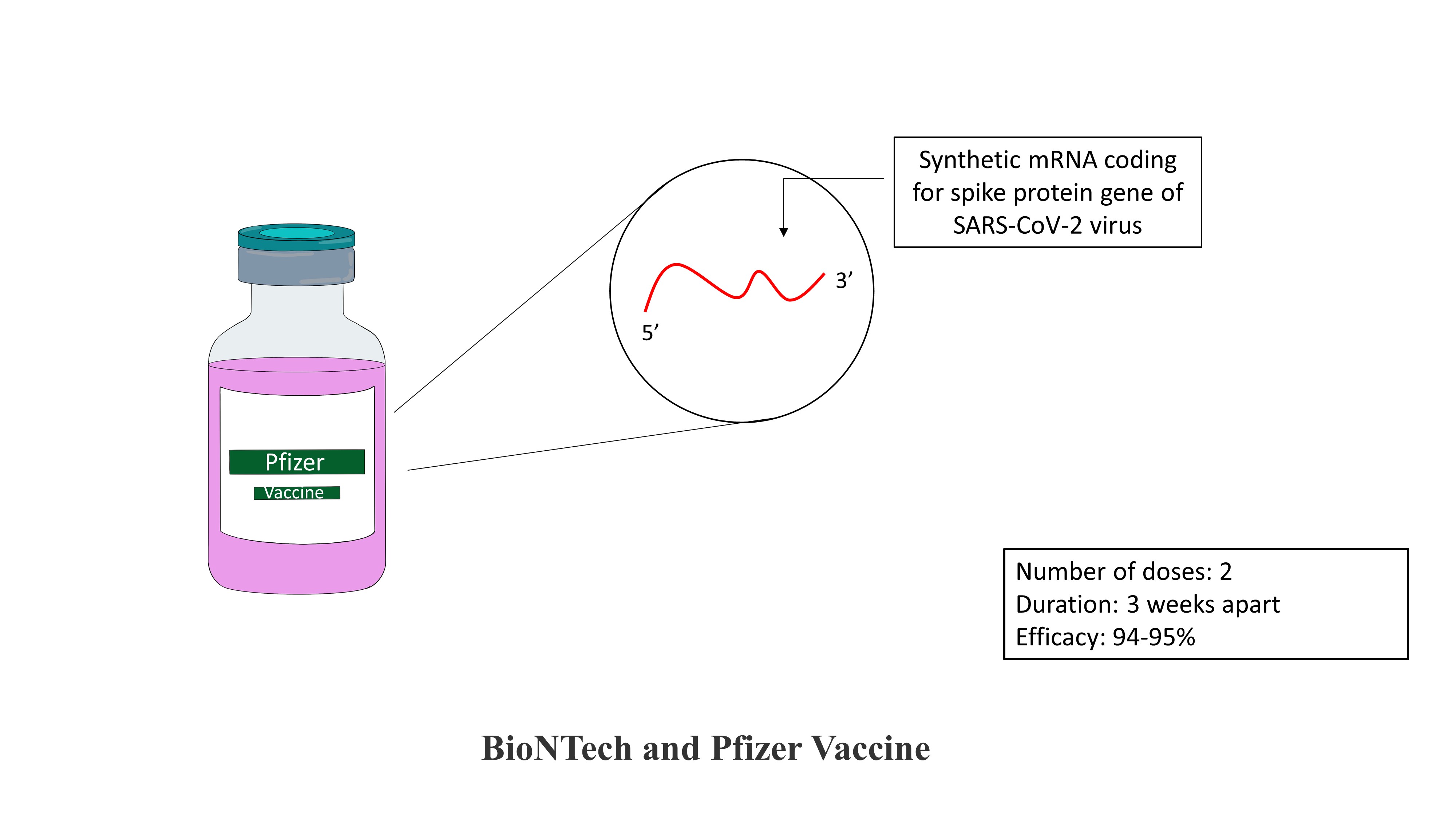
BioNTech and Pfizer Vaccine:
BioNTech and Pfizer vaccine against covid-19 is a jointly developed vaccine. The vaccine is an mRNA vaccine. The vaccine is composed of synthetic mRNA with a coding sequence of a gene for the spike protein of the SARS-COV-2 virus. The synthetic mRNA is enclosed in a lipid nanoparticle. These lipid nanoparticles fuse with a cell and deliver the synthetic mRNA in the cytoplasm of a cell, where it gets translated to produce an antigenic spike protein. The Pfizer vaccine is entirely safe as it does not contain any attenuated/inactivated virus or viral particle. Also, it does not alter the DNA as synthetic mRNA is only present in the cytoplasm, and it does not enter the nucleus. The vaccine is administered intramuscularly in two doses placed three weeks apart. The vaccine is found to be 94-95% efficient against the disease.
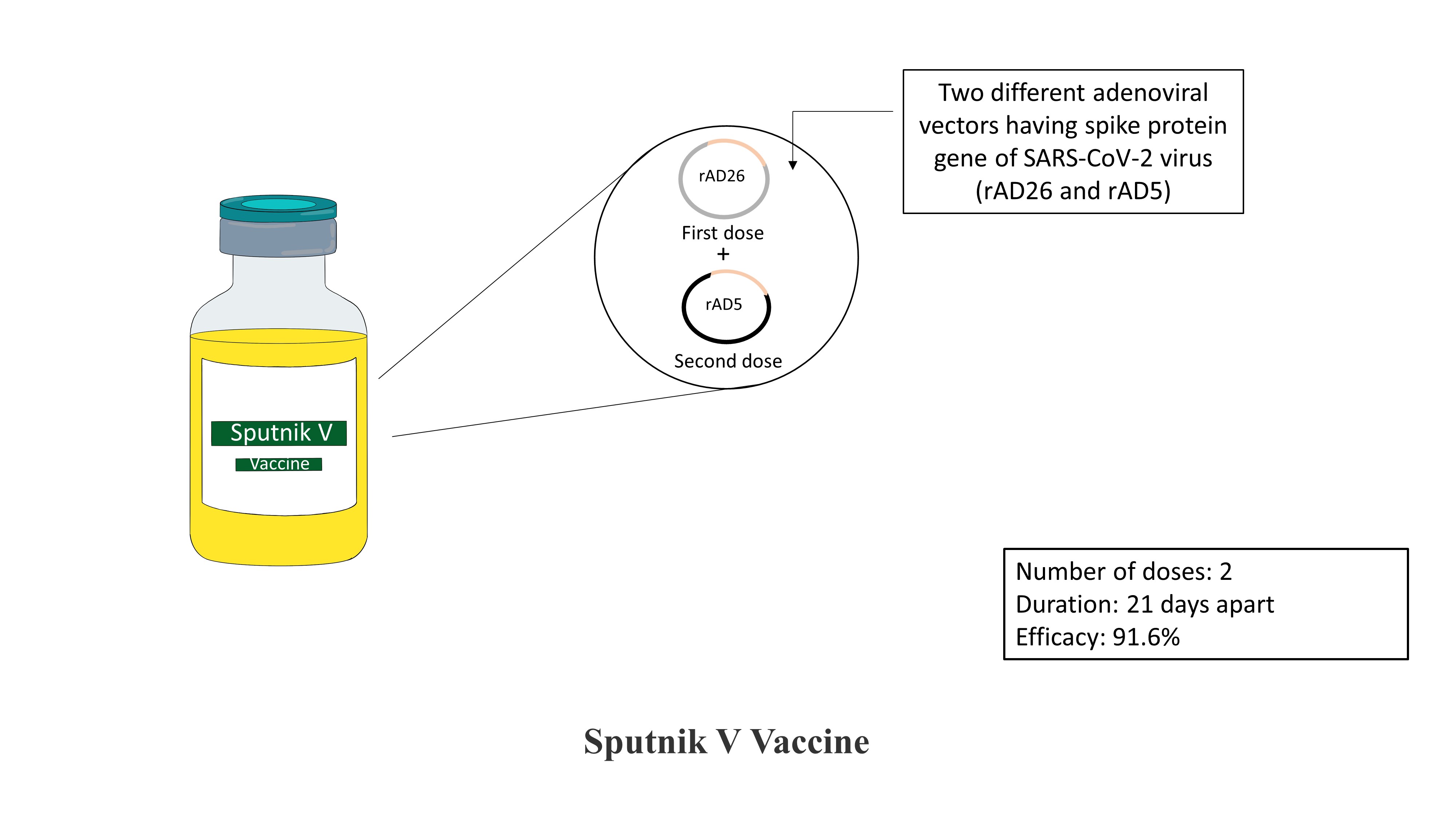
Sputnik V Vaccine:
The vaccine is developed by the Gamaleya National Centre for Epidemiology and Microbiology, Russia. This vaccine is also a viral vector vaccine, using an adenoviral vector as a carrying system for the gene coding for spike protein of SARS-COV-2. The vaccine is given in two doses, placed 21 days apart. A unique feature about this vaccine is that this vaccine uses two vector system. The first dose is a viral vector termed as rAD26 carrying a gene for spike protein, while the second dose is a viral vector termed as rAD5, also carrying the gene for spike protein. It has been reported that using such two vectors approaches the immune response generated is heightened compared to when only one type of vector is used. The efficacy of the vaccine reported via randomized trials is 91.6%.
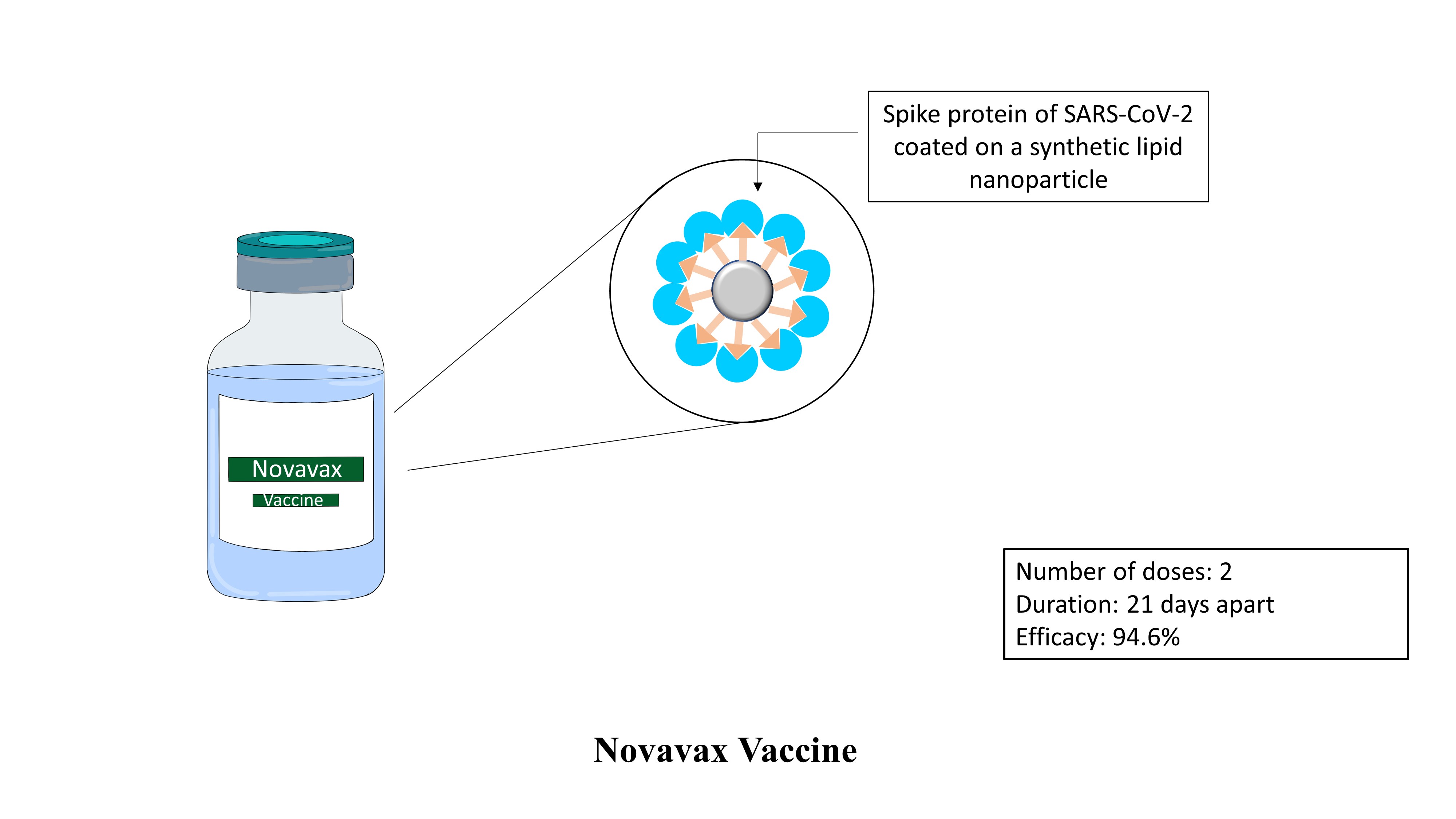
Novavax Covid-19 vaccine:
Novavax covid-19 vaccine termed NVX-CoV2373 is developed jointly by Novavax and the Coalition for Epidemic Preparedness Innovations (CEPI). This vaccine candidate is a unique vaccine candidate that can be classified as recombinant protein subunit vaccine and recombinant viral vector vaccine. Thus, a new term is used for this vaccine which is ‘recombinant nanoparticle vaccine’. The gene coding for Spike protein of coronavirus is cloned in a baculoviral vector. The viral vector is then cultivated in sf9 moth cells. Cells synthesize the spike proteins and display them on their surface. These spike proteins are then purified and assembled into a synthetic lipid nanoparticle. About 14 spike proteins are displayed onto a single nanoparticle. The vaccine is given in 2 doses intramuscularly 21 days apart. The vaccine is 94.6% effective against the original strain of the SARS-COV-2 virus.
Johnson and Johnson Vaccine:
This vaccine developed by Johnson and Johnson is also an adenoviral vector vaccine. The viral vector used is AD26 inserted with a gene coding for the spike protein of the virus. This vaccine is a single-dose vaccine. The efficacy of the vaccine is about 72%.
Moderna Vaccine:
The vaccine is primarily developed by Moderna in the United States of America. The Moderna vaccine is an mRNA vaccine consisting of a synthetic mRNA-1273, which codes for a spike protein. The synthetic mRNA is encapsulated in lipid nanoparticles. The dosage is in the form of two doses intramuscularly 28 days apart. The efficacy of the Moderna vaccine is 94.1% in clinical trials.